Basic Principles Fmoc Strong Phase Peptide Synthesis: A Functional Met…
Writer Monty Brigstocke
Date 24-11-11 16:55
4
0
본문
- Country : Brazil
- Item Name :
- Business Section : K4-eco
1925654624
- Email : montybrigstocke@sbcglobal.net
- Phone : 1925654624
- Message :
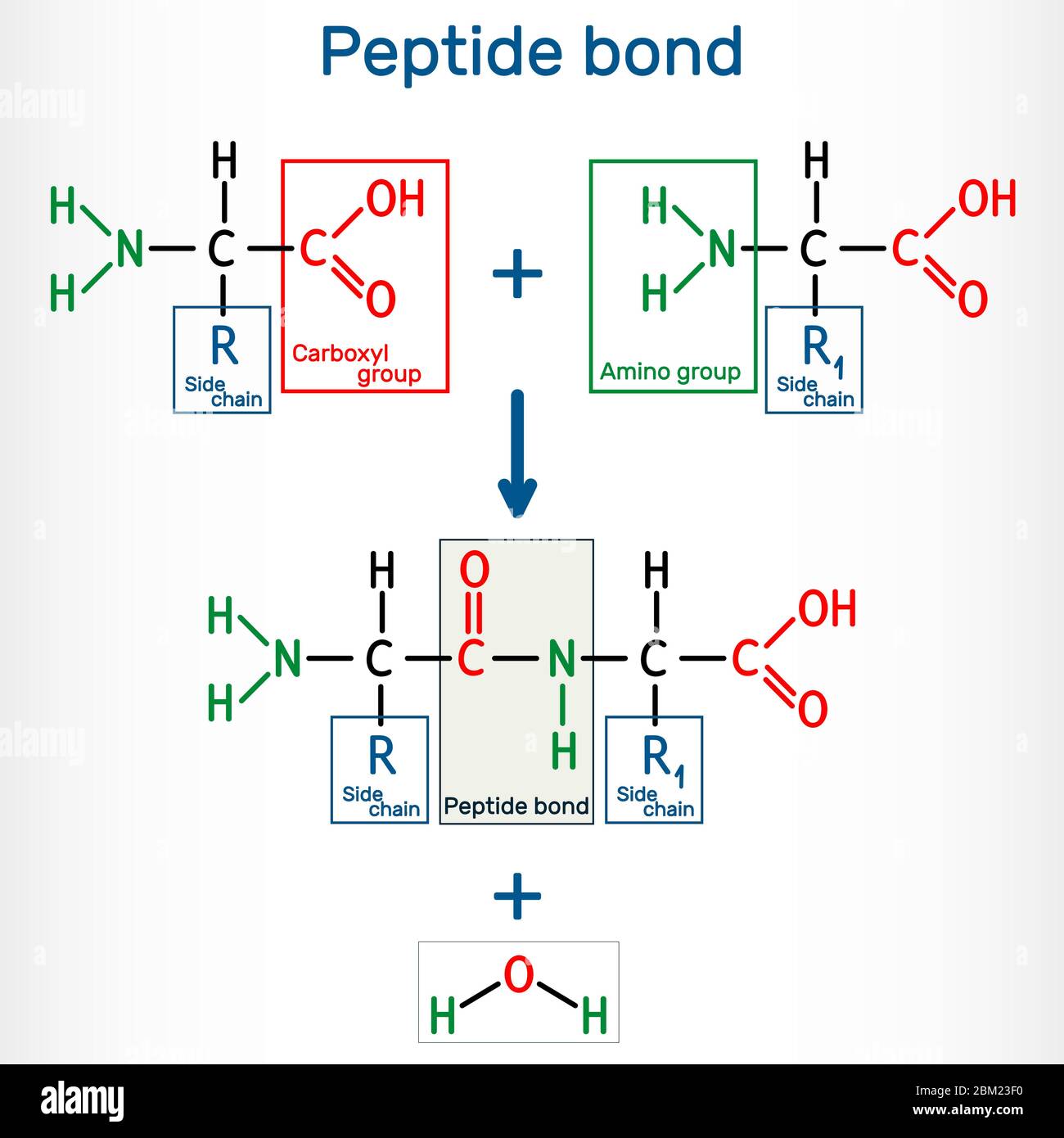
As currently talked about, peptide synthesis is the peptide bond formation in between 2 particular amino acids. Nonetheless, when it concerns a clear-cut meaning of peptides in biosciences, it simply indicates versatile little chain-like frameworks of amino acids. Furthermore, improvements in healthy protein chemistry and applications have actually established substantially in previous years. Today, the procedure has actually turned into one of the usual strategies in high-throughput research study and antibody manufacturing. Every one of the combining reagents reported up until now are applied in the common C-to-N structure of peptide sequences. Nonetheless, the procedure is based on making use of very harmful chemicals and the development of potentially eruptive intermediates.
The single advantage of the synthesis of peptides in the modern-day age is that besides developing peptides discovered in organic image, imagination and creativity can be suited to produce one-of-a-kind peptides and maximize wanted organic feedbacks. In the complying with areas, present patterns to raise sustainability of downstream handling of peptides are extensively reviewed. Viewpoints and challenges in the search for greener products and strategies are likewise offered.
An additional variable that boosts antimicrobial task of peptides is the replacement of the hydroxyl team (OH) of the incurable carboxyl moiety by a key amine (NH2), causing the development of a terminal amide team (Strøm et al., 2002b). According to the writers, the development of incurable amides raises the positive fee density as a result of removal of the adversely charged carboxyl teams (COOH). This as a result lessens the electrostatic repulsion between the antimicrobial peptides and the adversely charged phospholipids existing at the microbial membrane layer. Another description is that incurable amide groups could play a safety impact versus chemical assaults-- carboxypeptidases-- and, subsequently, enhance the life span of antimicrobial peptides (Strøm et al., 2002b). " Requiring the synthesis onto a diphasic solid phase system, we can enhance the efficient concentration of the synthesis, which is actually what drives expense. You can have a 5 cubic meter reactor, but if you can only load it up with grams of material since the product is insoluble after that it does not truly matter," he claims.
 Peptide Thioesters By Fmoc Spps
Peptide Thioesters By Fmoc Spps
Improvements are being continuously reported for peptide Quality control (QC) standards, synthesis time and unique synthetic targets. Topical peptide research study has actually added to a continual improvement and expansion of Fmoc SPPS applications. Also before synthesis, certain shielding teams are contributed to the pure, independent amino acids utilized to create peptides. These protecting intrigues are then eliminated from the lately presented amino acid (a process referred to as deprotection) shortly after coupling, allowing the succeeding amino acid to affix to the broadening peptide chain correctly. Upon completion of peptide synthesis, all remaining securing groups are cleared from the precursor peptides. Three common safeguarding teams are reviewed below depending on the Custom peptide quotation synthesis process.
A Simpler And More Cost-efficient Peptide Biosynthetic Approach Using The Truncated Gst As Service Provider For Epitope Mapping
In eukaryotes, phosphorylation takes place on serine, Peptide backbone modification threonine, tyrosine and histidine 161 deposits, whereas in prokaryotes, it is likewise observed on lysine and arginine 162. Cysteine racemisation accompanies base‐mediated activation techniques, such as those using phosphonium or uronium reagents 70, 71. Often, the prospective benefit surpasses the incredible cost of dealing with human mistakes.
EtOH has likewise greater elution strength with respect to MeOH, suggesting that much less EtOH is needed for the elution of the target at similar retention times. These factors to consider show the vital function played by solvents in making peptide manufacturing a greener truth. The solvents' impact on the atmosphere has additional reading social and economic results that cost concerning $1 billion per year in technologically innovative countries, together with results on ecological communities and health and wellness. For a solvent to be defined as "eco-friendly", it needs to adapt not just to EHS evaluations yet also to power need examinations.
 In summary, sortagging has a minimal range in CEPS as a result of the LPTXG motif sequence that should exist in the target peptide (there are no business peptides with that series) and the huge quantity of enzyme essential to obtain good conversions.
In summary, sortagging has a minimal range in CEPS as a result of the LPTXG motif sequence that should exist in the target peptide (there are no business peptides with that series) and the huge quantity of enzyme essential to obtain good conversions.
Developing greener synthesis processes is an inevitable need to transform the commercial landscape, generally in the pharmaceutical industry, right into a long-lasting, sustainable reality. In this context, the renaissance of peptides as clinical treatments, and the enforcement of extra rigorous sustainability needs by regulative firms, pressed chemists toward the intro of lasting procedures to prepare highly pure, energetic pharmaceutical components (APIs). These technologies are also approaching the intro of constant procedures that stand for among the most crucial targets for iterative procedures. This introduction goes over one of the most current efforts in making peptide chemistry greener. The comprehensive research studies that were executed on eco-friendly solvents, response conditions, complementary reagents and purification modern technologies in the peptide section can be valuable to various other fields of natural synthesis.
Peptide Synthesis
- Item Name :
- Business Section : K4-eco
1925654624
- Email : montybrigstocke@sbcglobal.net
- Phone : 1925654624
- Message :
As currently talked about, peptide synthesis is the peptide bond formation in between 2 particular amino acids. Nonetheless, when it concerns a clear-cut meaning of peptides in biosciences, it simply indicates versatile little chain-like frameworks of amino acids. Furthermore, improvements in healthy protein chemistry and applications have actually established substantially in previous years. Today, the procedure has actually turned into one of the usual strategies in high-throughput research study and antibody manufacturing. Every one of the combining reagents reported up until now are applied in the common C-to-N structure of peptide sequences. Nonetheless, the procedure is based on making use of very harmful chemicals and the development of potentially eruptive intermediates.
The single advantage of the synthesis of peptides in the modern-day age is that besides developing peptides discovered in organic image, imagination and creativity can be suited to produce one-of-a-kind peptides and maximize wanted organic feedbacks. In the complying with areas, present patterns to raise sustainability of downstream handling of peptides are extensively reviewed. Viewpoints and challenges in the search for greener products and strategies are likewise offered.
An additional variable that boosts antimicrobial task of peptides is the replacement of the hydroxyl team (OH) of the incurable carboxyl moiety by a key amine (NH2), causing the development of a terminal amide team (Strøm et al., 2002b). According to the writers, the development of incurable amides raises the positive fee density as a result of removal of the adversely charged carboxyl teams (COOH). This as a result lessens the electrostatic repulsion between the antimicrobial peptides and the adversely charged phospholipids existing at the microbial membrane layer. Another description is that incurable amide groups could play a safety impact versus chemical assaults-- carboxypeptidases-- and, subsequently, enhance the life span of antimicrobial peptides (Strøm et al., 2002b). " Requiring the synthesis onto a diphasic solid phase system, we can enhance the efficient concentration of the synthesis, which is actually what drives expense. You can have a 5 cubic meter reactor, but if you can only load it up with grams of material since the product is insoluble after that it does not truly matter," he claims.
 Peptide Thioesters By Fmoc Spps
Peptide Thioesters By Fmoc Spps Improvements are being continuously reported for peptide Quality control (QC) standards, synthesis time and unique synthetic targets. Topical peptide research study has actually added to a continual improvement and expansion of Fmoc SPPS applications. Also before synthesis, certain shielding teams are contributed to the pure, independent amino acids utilized to create peptides. These protecting intrigues are then eliminated from the lately presented amino acid (a process referred to as deprotection) shortly after coupling, allowing the succeeding amino acid to affix to the broadening peptide chain correctly. Upon completion of peptide synthesis, all remaining securing groups are cleared from the precursor peptides. Three common safeguarding teams are reviewed below depending on the Custom peptide quotation synthesis process.
A Simpler And More Cost-efficient Peptide Biosynthetic Approach Using The Truncated Gst As Service Provider For Epitope Mapping
In eukaryotes, phosphorylation takes place on serine, Peptide backbone modification threonine, tyrosine and histidine 161 deposits, whereas in prokaryotes, it is likewise observed on lysine and arginine 162. Cysteine racemisation accompanies base‐mediated activation techniques, such as those using phosphonium or uronium reagents 70, 71. Often, the prospective benefit surpasses the incredible cost of dealing with human mistakes.
EtOH has likewise greater elution strength with respect to MeOH, suggesting that much less EtOH is needed for the elution of the target at similar retention times. These factors to consider show the vital function played by solvents in making peptide manufacturing a greener truth. The solvents' impact on the atmosphere has additional reading social and economic results that cost concerning $1 billion per year in technologically innovative countries, together with results on ecological communities and health and wellness. For a solvent to be defined as "eco-friendly", it needs to adapt not just to EHS evaluations yet also to power need examinations.
 In summary, sortagging has a minimal range in CEPS as a result of the LPTXG motif sequence that should exist in the target peptide (there are no business peptides with that series) and the huge quantity of enzyme essential to obtain good conversions.
In summary, sortagging has a minimal range in CEPS as a result of the LPTXG motif sequence that should exist in the target peptide (there are no business peptides with that series) and the huge quantity of enzyme essential to obtain good conversions.Developing greener synthesis processes is an inevitable need to transform the commercial landscape, generally in the pharmaceutical industry, right into a long-lasting, sustainable reality. In this context, the renaissance of peptides as clinical treatments, and the enforcement of extra rigorous sustainability needs by regulative firms, pressed chemists toward the intro of lasting procedures to prepare highly pure, energetic pharmaceutical components (APIs). These technologies are also approaching the intro of constant procedures that stand for among the most crucial targets for iterative procedures. This introduction goes over one of the most current efforts in making peptide chemistry greener. The comprehensive research studies that were executed on eco-friendly solvents, response conditions, complementary reagents and purification modern technologies in the peptide section can be valuable to various other fields of natural synthesis.
Peptide Synthesis